How Do You Know What Order a Reaction Is
xiii.four: Determining Order of Reaction
Rate laws describe the relationship between the rate of a chemical reaction and the concentration of its reactants. In a rate law, the rate constant k and the reaction orders are determined experimentally past observing how the rate of reaction changes as the concentrations of the reactants are changed. A common experimental approach to the determination of rate laws is the method of initial rates. This method involves measuring reaction rates for multiple experimental trials carried out using different initial reactant concentrations. Comparing the measured rates for these trials permits determination of the reaction orders and, after, the rate constant, which together are used to formulate a rate police.
The charge per unit of a reaction, for instance, involving nitric oxide with ozone [NO (thousand) + O3 (g) ⟶ NO2 (one thousand) + Oii (thousand)] tin can be adamant from the experimental data of method of initial rates, in the laboratory.
| Trial | [NO] (mol/L) | [O3] (mol/L) | Δ[NO2]/Δt (mol/Fifty·s) |
| one | ane.00 × 10−6 | 3.00 × ten−6 | 6.60 × x−5 |
| 2 | 1.00 × x−6 | half-dozen.00 × x−six | i.32 × ten−4 |
| 3 | one.00 × 10−6 | 9.00 × 10−6 | 1.98 × 10−four |
| 4 | two.00 × ten−vi | 9.00 × x−6 | 3.96 × 10−four |
| 5 | 3.00 × x−vi | nine.00 × ten−6 | five.94 × 10−4 |
From the rate data, a generic rate police; rate = g[NO] m [O3] n is formulated. The values of the reaction orders grand and northward, and charge per unit constant 1000 are adamant from the experimental data using a three-role process:
In pace i, the value of m is determined from the data in which [NO] varies, and [O3] is constant. In trials 3, 4 and 5, [NO] varies while [O3] remains constant. When [NO] doubles from trial three to 4, the rate doubles, and when [NO] triples from trial 3 to v, the rate also triples. Thus, the rate is also directly proportional to [NO], and m in the rate law is equal to i.
In step ii, the value of n is adamant from information in which [O3] varies, and [NO] is abiding. In trials 1,2 and 3, [NO] is constant and [O3] varies. The reaction rate changes in direct proportion to the change in [Othree]. When [O3] doubles from trial ane to 2, the charge per unit doubles; when [O3] triples from trial ane to 3, the rate increases besides triples. Thus, the rate is direct proportional to [Othree], and n is equal to 1. The rate law is thus: charge per unit = k [NO]1 [O3]1 = k [NO][O3]
In step 3, the value of thousand is determined from ane set of concentrations (for case, the data from trial i) and its respective charge per unit.
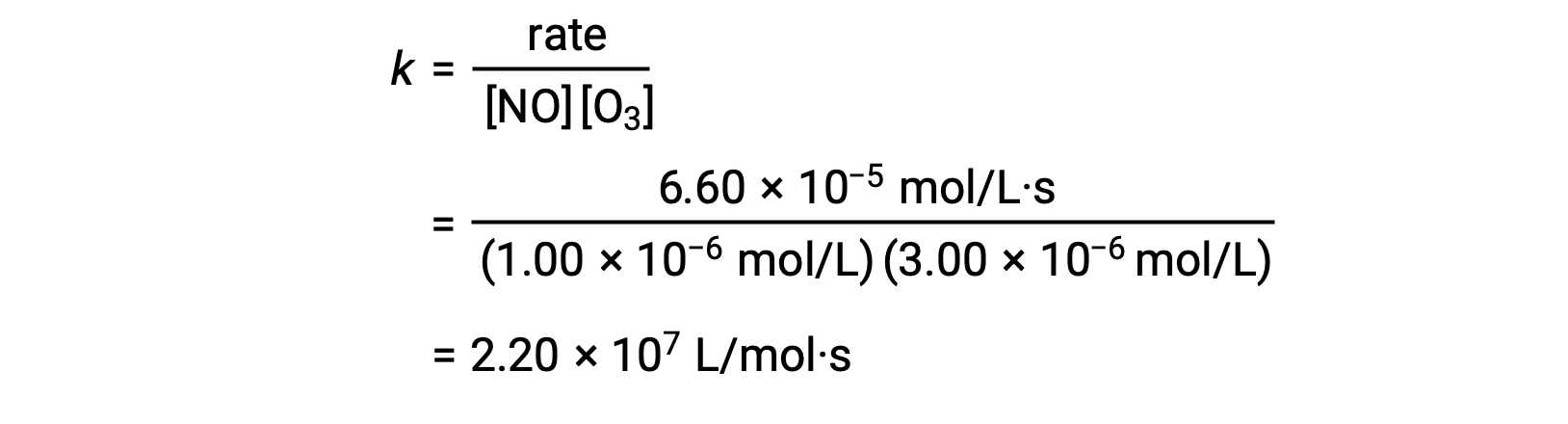
In reactions where the data from the method of initial rates does non directly imply the relation between initial concentrations and initial rates, a calculation involving the ratio of rate laws can be employed to calculate the reaction club and charge per unit abiding.
For instance, the general charge per unit law for the reaction 2 NO (yard) + Cl2 (chiliad) ⟶ 2 NOCl (grand) is expressed as: rate = k [NO] m [Cltwo] n .
The data from the method of initial rates are:
| Trial | [NO] (mol/L) | [Cl2] (mol/L) | Initial Rate (mol/L·southward) |
| 1 | 0.10 | 0.10 | 0.00300 |
| 2 | 0.x | 0.15 | 0.00450 |
| iii | 0.15 | 0.10 | 0.00675 |
The values of one thousand and north can be determined from the experimental information using an algebraic arroyo, following which the value of chiliad is determined.
In pace 1, the value of chiliad is adamant from the data in which [NO] varies and [Cl2] is abiding. A ratio of rate laws is expressed by substituting data from 2 different trials (for instance trial iii and trial i).
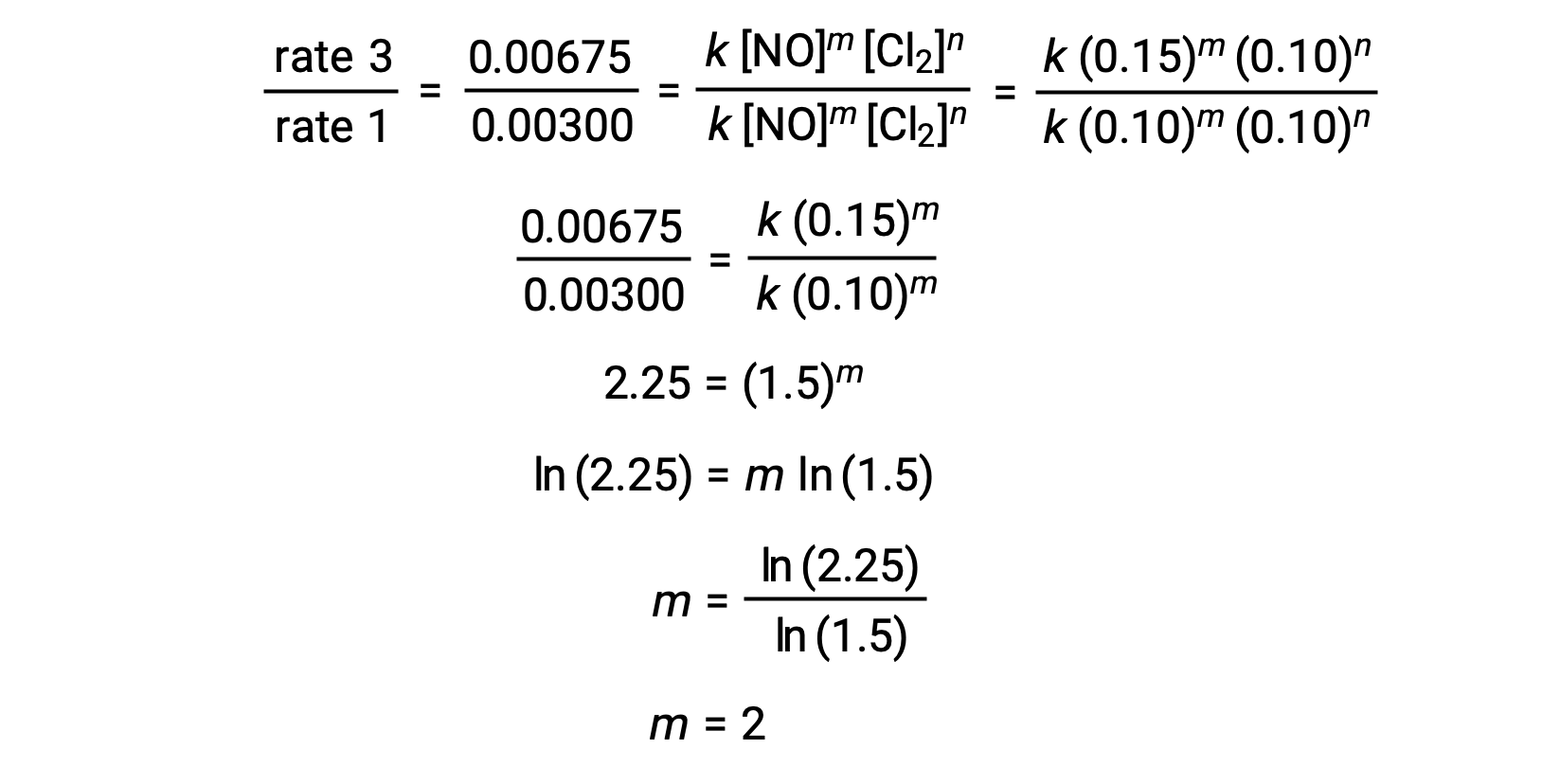
In pace two, the value of northward is determined from data in which [Cl2] varies, and [NO] is constant.
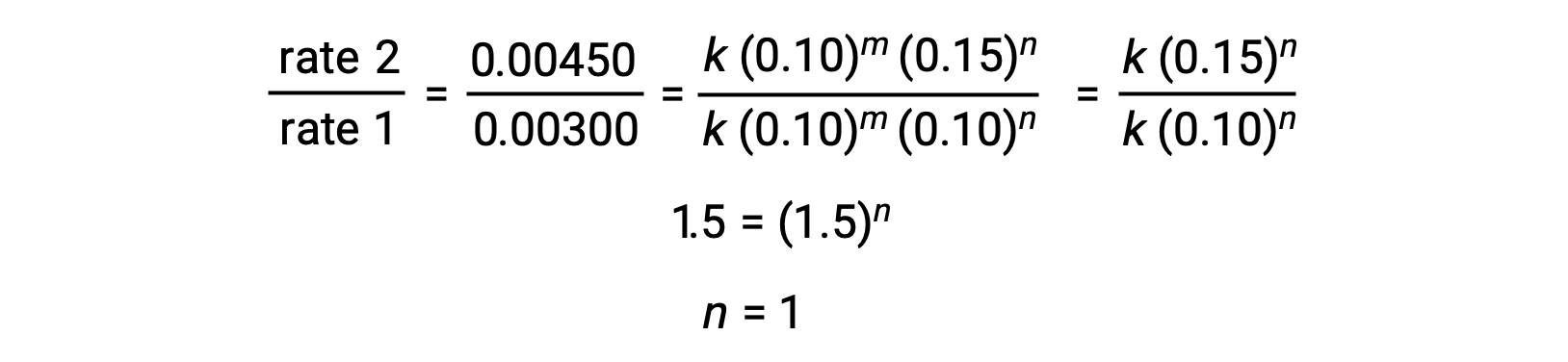
Using the computed values of m and due north the rate police force is expressed as rate = thousand [NO]2 [Cl2].
In step 3, the numerical value of the charge per unit constant chiliad is determined with advisable units. The units for the rate of a reaction are mol/L·s. The units for m is concluded by substituting the units of all other parameters in the rate police. In this case, the concentration units are mol3/L3. The units for k should exist Lii/mol2·s and so that the rate is in terms of mol/L·due south. The value of k is determined in one case the charge per unit law expression has been solved, by simply substituting the values from any of the experimental trials (for example trial one).
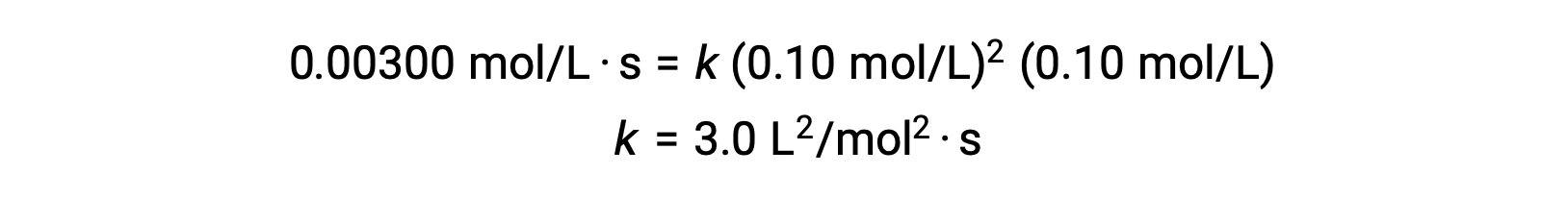
Reaction Gild and Rate Constant Units
In some reactions, the reaction orders in the charge per unit police force happen to be the aforementioned equally the coefficients in the chemical equation for the reaction. This is merely a coincidence and very ofttimes non the case. Rate laws may showroom fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in the concentration of one reactant causes a subtract in reaction rate. Rate laws are adamant by experiment only and are not reliably predicted past reaction stoichiometry.
The units for a rate abiding volition vary every bit appropriate to suit the overall society of the reaction. The unit of the rate constant for a nix-order reaction is mol/L·southward (or M/s) and that for a offset-order reaction is ane/s. The unit of the rate constant for a 2nd-club reaction is 50/mol·due south (or ane/M·s) and that for a third-order reaction is Ltwo/mol2·s. Although the specific units for concentration and fourth dimension are indicated as (mol/L) and (due south), whatsoever other valid units can be used to represent the properties of concentration and time.
This text is adjusted from Openstax, Chemistry 2e, Section 12.3: Charge per unit Laws.
spellmanhatinarthady.blogspot.com
Source: https://www.jove.com/science-education/11376/determining-order-of-reaction
Post a Comment for "How Do You Know What Order a Reaction Is"